
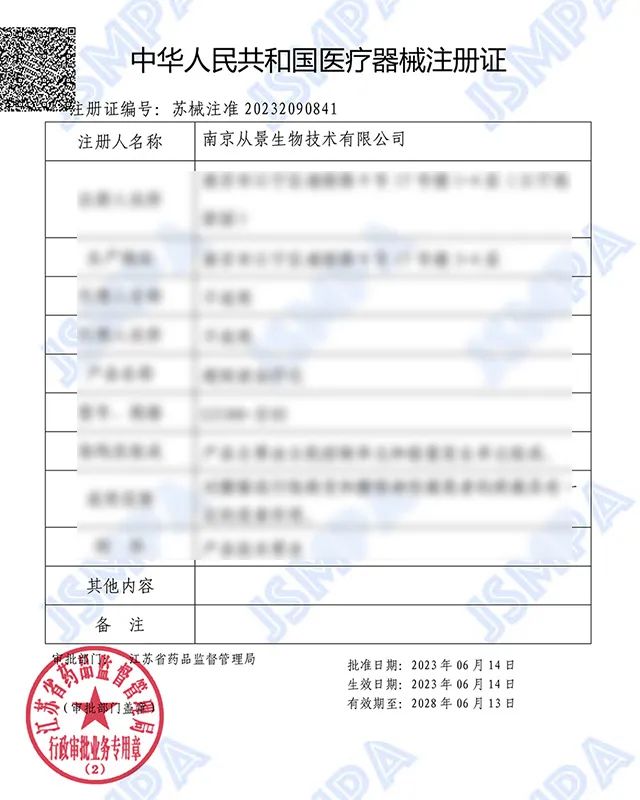
On June 15, 2023, after going through the process of product design and development, safety risk analysis, registration testing, clinical effectiveness test, quality system assessment, review and approval, Nanjing Conjing Biotechnology Co., LTD. (hereinafter referred to as "CONGJING Group") independently developed the "ultra-short wave therapeutic instrument CJ180-Ⅱ02" (market abbreviation: Moxibustion stool) obtained the national Class II medical device registration certification!
As of June 2023, there are only 19 ultra-short wave products (approved in the form of domestic medical devices) that have been approved for medical device registration in China (CONGJING Group accounts for 4). CONGJING Group in China traditional medicine and engineering integration of ultra-short wave therapeutic instrument (acupuncture stool) products to be approved the second class medical device registration certificate strongly proves that Congjing's scientific research strength and product safety and effectiveness in the medical field and the market has been fully recognized! It also proves that the clinical value of Congjing products can meet the professional requirements of the national medical device regulatory authorities, and adds a strong ink to the strategic layout of the group's products!
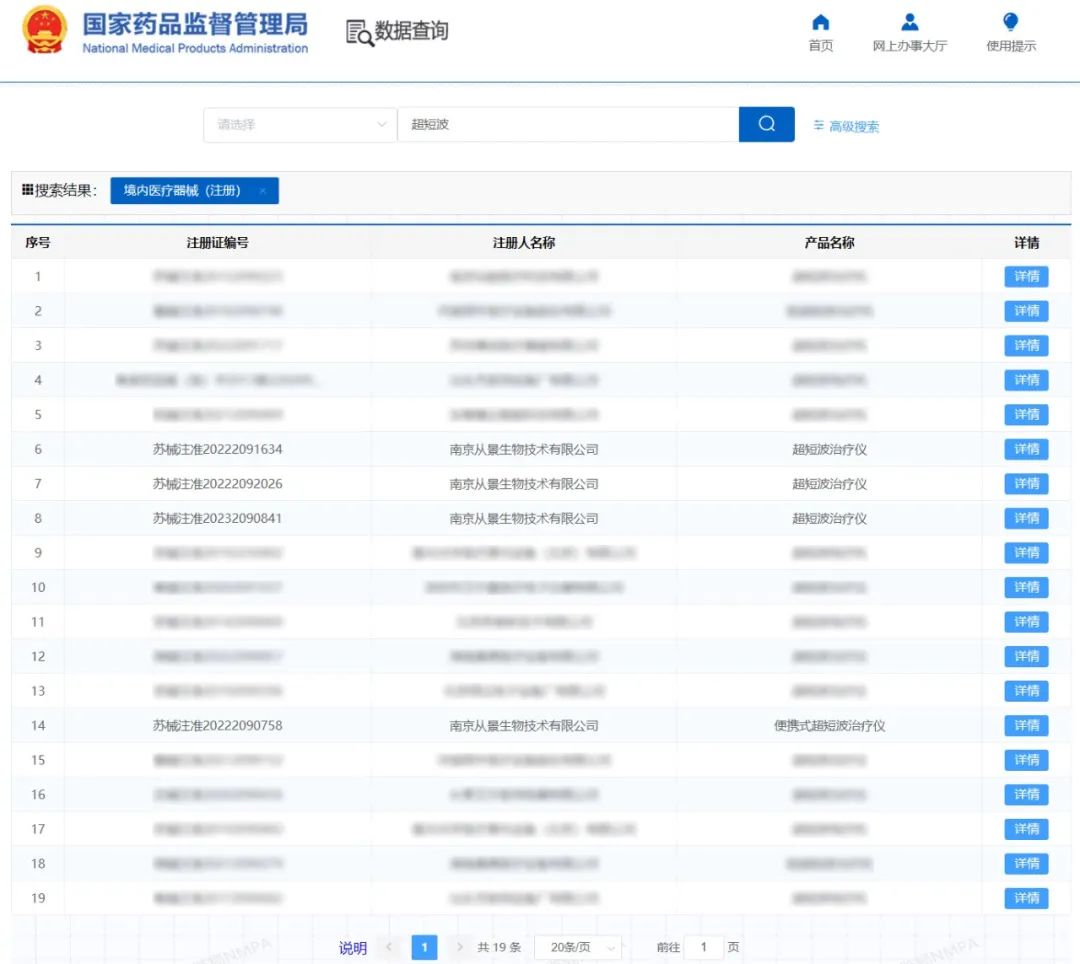
So far, CONGJING has achieved full leading advantages in market access such as tumor hyperthermia and rehabilitation physiotherapy, and is a rare group enterprise in the market with three medical device registration certificates and two medical device registration certificates, and it continues to apply every year, and will involve clinical applications in oncology, rehabilitation, internal medicine, surgery and other departments in the future.
Contact: Chyibo
Phone: 0086-13776611575
Tel: 0086-13776611575
Email: vip@congjing.net.cn
Add: Building 15, Phase II, Liandong U Valley, Jiangning High-tech International Enterprise Port, No. 7 Tonglian Road, Jiangning District,Nanjing China.
We chat
